COVID-19 news and updates
St. Luke’s contributes to national COVID-19 virus treatment research
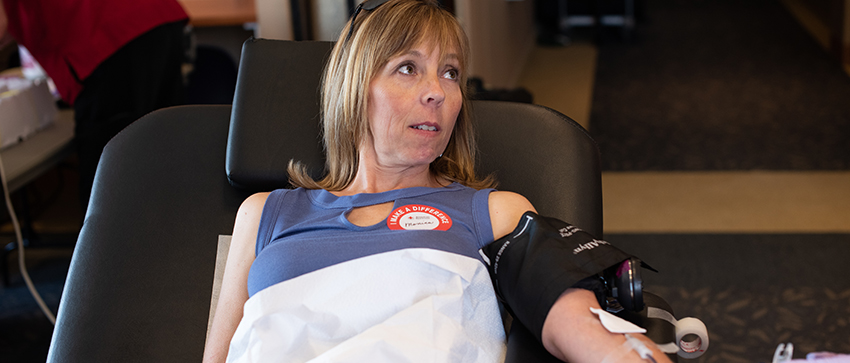

Caring for patients infected with the novel coronavirus is an ongoing challenge.
And without proven treatments, providers are turning to methods that have been effective in the past, including convalescent plasma transfusion – an approach used successfully during another pandemic 100 hundred years ago.
St. Luke’s now can treat COVID-19 virus patients with convalescent plasma through participation in Mayo Clinic’s Expanded Access Program. Convalescent plasma found favor as a treatment during the Spanish flu pandemic that started in 1918 and blazed across the world through 1920, infecting about a third of the population.
COVID-19 convalescent plasma (CCP) comes from the blood of patients who have recovered from the disease and contains antibodies that help fight infection. It has been shown to work in small numbers of patients in China, South Korea and Europe, and has seen favorable results in the first patients treated with it in New York.
“When we get far enough along (with a treatment) that we determine its benefits outweigh the risk of any potential negative impact to the patient, the FDA can approve emergency use for an investigational drug,” said Anne Burkey, St. Luke’s blood management quality program specialist.
The Mayo Clinic took the lead to establish an expanded access program that allows participating organizations to treat patients under its FDA permissions in exchange for sharing patient-result data. St. Luke’s is one of more than 2,000 sites contributing data. More than 3,000 patients nationwide have already received transfusions.
St. Luke’s Dr. David Hinchman, a cardiologist in Boise, is the physician investigator, acting as the liaison between the infectious disease department, ordering providers, Mayo Clinic investigators and the FDA. He heard about the treatment’s early success and played a critical role in orchestrating St. Luke’s involvement in the national research effort.
"This is an unproven treatment, so each patient needs to be reported to the FDA,” Dr. Hinchman said. “The characteristics of the patient, how they did and what the outcomes are will be shared, so we can find out what needs to be done next from what we’ve learned.”
The Mayo Clinic program lays out stringent guidelines for providers administering COVID-19 convalescent plasma. From the data gathered, researchers will determine the most effective dosage, characteristics of patients that experience best results, optimal treatment timing and more.
One to two units of plasma transfused to the most critically ill, acute patients is the initial baseline recommendation.
“We’re moving so quickly, so they’re saying start with this,” Dr. Hinchman said. “We’re going to learn as we go.”
Health-care providers have tried a host of drugs under investigation for COVID-19 treatment, including remdesivir, hydroxychloroquine, chloroquine and azithromycin. COVID-19 convalescent plasma appears to be the most promising solution, which is why St. Luke’s joined the national effort.
“In the early punch that COVID-19 brought to our nation and community, this was one of the first opportunities where we could come together to do something,” St. Luke’s Medical Director for Research Dr. James Loveless, a rheumatologist in Boise, said.
“Our role is to support frontline providers and patients in our community. We decided to be proactive, so that if we got a call that providers wanted CCP, we were ready.”
St. Luke’s clinical research, blood management, infectious disease, transfusion medicine, informatics, information health technology and education departments, as well as many interdisciplinary providers, collaborated to take part in the program.
“It’s been a tremendous coordinated effort,” Dr. Hinchman said. “To date, it still looks to be one of the most promising treatments.”
Providing needed support
The American Red Cross has begun collecting convalescent plasma in some of the United States’ most populous and hardest-hit cities.
Those interested in contributing must have first tested positive for COVID-19 while exhibiting symptoms, be fully recovered and have a subsequent negative test result. The American Red Cross in Boise has begun compiling a list of potential donors.
Sign up here for consideration and to be contacted for eligibility screening when collection becomes available. COVID-19 convalescent plasma donations will only be accepted at the American Red Cross in Boise, though eligible donors outside the Treasure Valley are encouraged to register.
“Individuals who have recovered from COVID-19 for at least 28 days and meet all standard donor criteria should consider making an appointment to donate, preferably COVID-19 convalescent plasma, but if that is not an option, they should consider making an appointment as a standard blood donor,” said Dr. Walter Kelley, division medical director at the American Red Cross.
“All donors save lives!”
About The Author

Alexis Bennett is a consultant for St. Luke's Center For Community Health.


